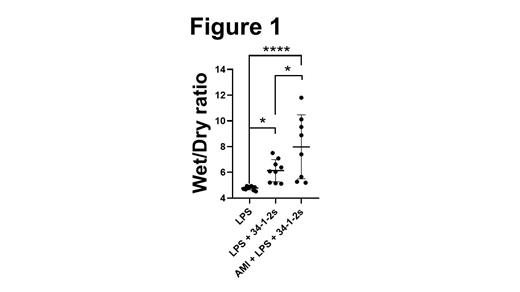
Transfusion-related acute lung injury (TRALI) is a leading cause of transfusion-related fatalities with a 5 to 14% mortality rate. Symptoms range from mild dyspnea, cough, fever to full-blown respiratory failure and severe hypoxemia within 6 hours from the transfusion. To date, the pathogenesis is not fully understood, but it appears that TRALI requires two hits in order to be initiated; the first-hit is the patient's clinical condition often reflected by a state of inflammation while the second hit is the transfusion itself (often containing anti-donor antibodies). Several reports have described platelet involvement in antibody-mediated TRALI, however, there are significant controversies that have arisen as to whether intact platelets actually play a role or not. On the other hand, platelet extracellular vesicles (PEV) have been found to accumulate in stored platelet units and are positively correlated with significant adverse transfusion reactions. Furthermore, in a murine antibody-independent model of TRALI, it was recently demonstrated that PEV increase in stored murine platelet concentrates and are associated with TRALI-induction via excess ceramide production. We have used an in vivo murine model of TRALI where liposaccharide (LPS)-primed BALB/c mice are injected with a murine monoclonal anti-MHC class I antibody (34-1-2s). This antibody-mediated TRALI model exhibits severe acute lung injury that closely mimics the human condition. We attempted to understand what role recipient PEV may play in antibody-dependent TRALI. Male BALB/c mice were administered LPS ip (0.1 mg/kg) 18 hrs prior to initiating TRALI. At time zero, the mice are injected iv with 34-1-2s (1 mg/kg) and subsequently, TRALI manifestations were monitored every 30 min, and at 90 min post-injection, blood and lungs were harvested for the indicated measurements. Results show that compared with isotype-control treated mice, animals administered 34-1-2s had significantly elevated lung Wet/Dry (W/D) ratios (measure of lung edema, Figure 1a) and pulmonary neutrophil accumulation. The TRALI mice had significant thrombocytopenia and elevated levels of PEV in their plasma as detected by High-sensitivity Flow Cytometry (Hs-FCM). To determine the role of in vivo generated PEV in antibody-mediated TRALI, mice were first administered amitriptyline (AMI), a potent inhibitor of PEV formation. AMI treatment significantly reduced plasma PEV levels, but after administration of 34-1-2s, TRALI responses (lung edema) were significantly enhanced in the AMI-treated mice (Figure 1). To determine if this effect was due to PEV-derived serotonin, mice were next treated with Prozac to deplete platelet/PEV stores of serotonin. Prozac-treated mice had normal levels of PEV after 34-12s administration and TRALI induction was not affected. Our results suggest that in antibody-mediated TRALI, recipient generated PEV play a suppressive role that is independent of serotonin. Further studies are underway to determine the mechanism(s) of PEV-mediated TRALI suppression. Thus, modulating PEV formation in recipients may be an effective therapy for reducing TRALI reactions.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal